Many relevant diagnostic signs are not performed deliberately by the examiner or by the patient at the examiner’s direction. They are observed as the patient reacts to their condition. Fortin’s finger sign, Minor’s sign, and Vanzetti’s sign are three examples of this principle.
Toxic Metal Elimination by Mineral Substitution
Lead, mercury, cadmium, and aluminum are toxic metals to which we are becoming increasingly exposed. Lead is considered the most dangerous due to the extent that it is distributed in the environment as a polluting element. For over a half-century, tetraethyl lead was added to gasoline to make the manufacture of fuel less expensive, and drivers distributed lead in the form of dust over the roads and byways throughout the world.
The magnitude of the problem is shown by the fact that over 400,000 tons of industrial lead are dumped into the atmosphere every year. This lead settles back into the soil, gardens, and farm lands so that it enters directly into the food supply. Estimates by the American Academy of Medical Preventics suggest that approximately 38 million Americans are being slowly poisoned by lead.
Cadmium
Cadmium as a pollutant is becoming a serious health problem due to its extensive use in the rubber and metal manufacturing industries. Cadmium plating is used on many metals to prevent rust, and cadmium added to automobile tires improves the quality of the rubber. Tobacco contains significant amounts of cadmium, with one pack of cigarettes containing approximately 23 micrograms of the toxic metal.
Unfortunately, the major portion of cadmium in tobacco is volatilized, and passes out in the smoke and is inhaled. Since cadmium interferes with the function of several enzymes, especially those involving zinc, copper, and selenium, it is obvious that cadmium in excess can cause disease. Most notable is hypertension, and kidney and liver damage. Ophthalmologists have indicated that a seemingly disproportionately high percentage of patients with intransigent glaucoma have elevated cadmium levels compared to others.
Mercury
Mercury is a third heavy metal which is frequently used in industry, and until very recently, the industrial waste material was dumped into our rivers, lakes, and the ocean. As a result, fish and seafood have become significantly contaminated with mercury, necessitating bans on their sale for food. Mercury is used in industrial fungicides, and in compounds used to preserve seed during the germination process. Some of this mercury also enters the food chain. Because of their toxicity to life forms, mercury compounds are commonly used as preservatives in solutions, most notably eye and nose drops.
Allergy and toxicity from such compounds is very common; so common, in fact, that manufacturers of drops for contact lens wearers have been forced to produce products without these preservatives. Mercury vapor is so toxic that even the breaking of a single mercury oral thermometer inside a submarine so large that 144 men live and work in it, will raise the mercury levels within the atmosphere to intolerable levels.
Although the American Dental Association argues that the mercury used in dental amalgams is permanently trapped within the filling, the fact is , it is not. Approximately 50 percent of the mercury in dental amalgams is slowly released over a period of a decade or so. Several nations, recognizing the danger, have already outlawed the use of mercury in dental repairs. Although the American Dental Association has waged a strong battle to discourage dentists from informing patients of the dangers, they have not been so timid about informing dentists and their employees. In their newspaper they warned, "Handling amalgams requires extreme caution and a 'no touch' technique should be employed." (But it's alright to leave it in your mouth!)
Aluminum
A fourth toxic metal of some concern, and some controversy, is aluminum. Aluminum is well absorbed and, fortunately, generally well excreted by the normal individual. However, some individuals appear to retain higher amounts of aluminum, and more specifically seem to admit aluminum into the normally isolated circulation of the brain; aluminum has thus been implicated in Alzheimer's Disease, Parkinson's Disease, and the dementia of dialysis.
Although small amounts of aluminum do erode from the surface of aluminum pots and pans, by far the largest contribution to dietary aluminum is the use of aluminum compounds in baking powder, flowing agents in salt, and in processing pickles, white flour, and other prepared food. Antiacid tablets and liquids containing aluminum are probably the major dietary source for many individuals. If you use antiacid, check the label to avoid products using aluminum. Antiacids using calcium and magnesium are strongly preferred in every case.
Potential Solution
Since our exposure to these toxins is going to remain constant throughout several life spans, regardless of our success in preventing additonal pollution of our environment, any reasonable method that might reduce tissue loads of these toxic metals could prove beneficial. Obviously, all of these metals can be eliminated from the body with appropriate intravenous chelation therapy, but this can prove to be expensive for some ($5,000 per patient), plus lost wages and time.
We decided to see if what we had observed could be proven with a clinical test of patients with levels of toxic metals in their system. The object was to reduce or eliminate the concentration of the toxic metals by a simple home-administered, but doctor supervised, protocol.
In order to assess the effectiveness of any method, a reliable test is necessary. Fortunately, the relatively inexpensive hair analysis performed by a reliable laboratory is the most effective test for tissue levels of the toxic metals. This is especially true of lead, cadmium, and mercury which disappear rapidly from the blood even after being administered by intravenous injection, so the blood levels reflect only the effect of an immediate exposure. Hair analysis, on the other hand, reflects the average concentration of the metal during a fairly recent period of exposure.
Procedures
Individuals with elevated levels of lead, mercury, or cadmium were selected for the study and a blood analysis of the specific mineral was performed in a sizable percentage of those in the study. However, repeat blood studies showed only modest decreases of blood levels as predicted, which confirmed the lack of value of blood studies in chronic heavy metal poisoning. Therefore, it was decided to utilize hair analysis as a means of determining levels of toxicity. Prior to administration of the test substance, hair samples from the nape of the neck were procured and analyzed.
Test Substance
Patients were treated with one ounce of Formula ACX three times daily with food. Once treatment was begun, the hair analysis was repeated at approximately three-month intervals, that frequency being impossible to totally control, since the hair can only be obtained conveniently when it has grown long enough to cut.
Formula ACX is a proprietary colloidal suspension of natural minerals found in humic shale. The product supplies over 40 grams of minerals per quart of product. In addition, it contains one gram of vitamin C per ounce, a broad spectrum of vitamins and free amino acids with supplemental amounts of calcium, magnesium, and potassium.
Study Protocol
During this study it became apparent that there were many individuals with low levels of toxic metals on initial hair analysis, who after taking substantial amounts of Formula ACX on a regular basis for several weeks, were discovered to have high levels of these metals upon repeat hair analysis.
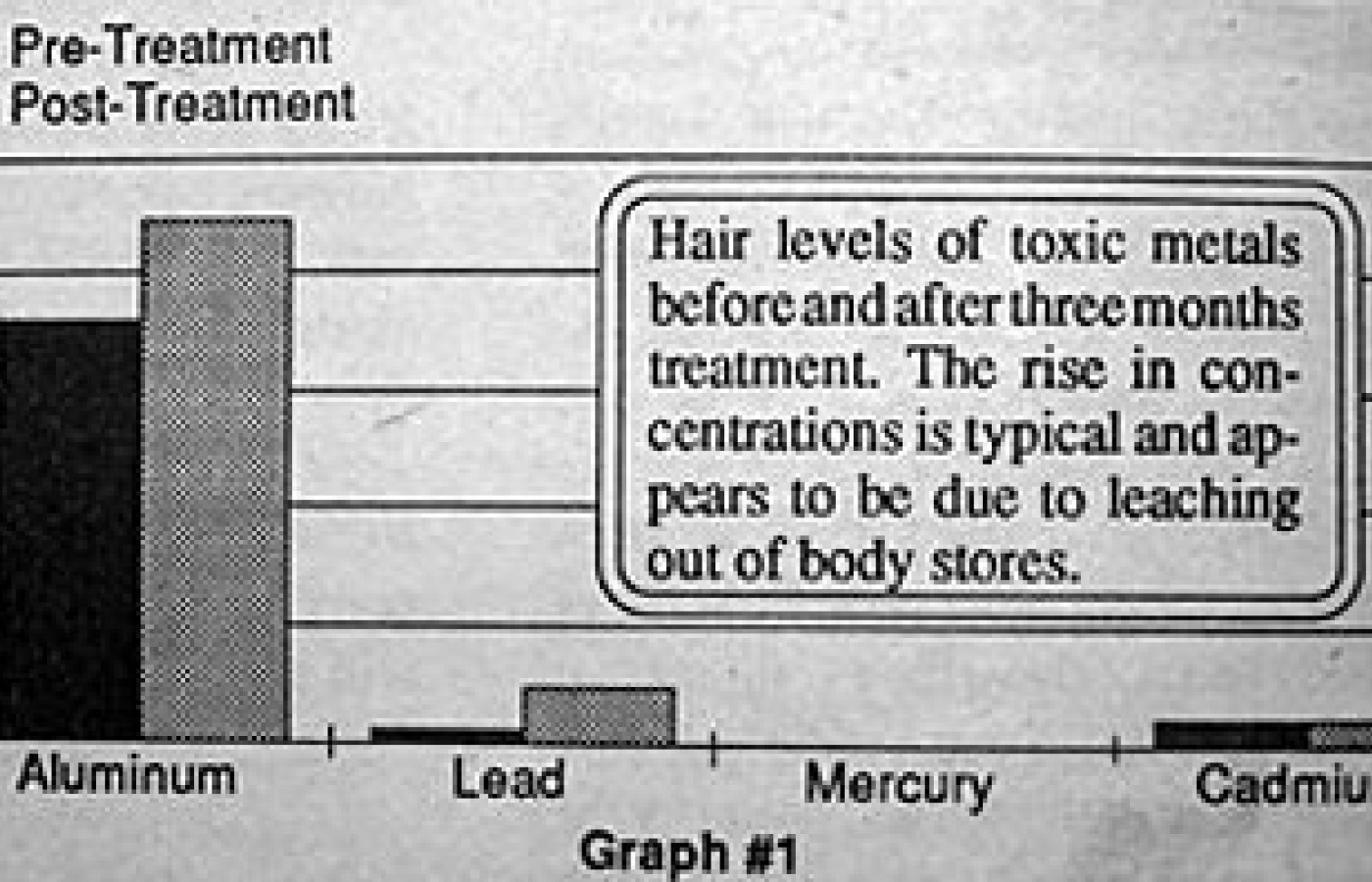
In fact, the three-month analysis demonstrated that, in most individuals with measurable levels of toxic metals at the onset of the study, an increase in toxic metals occurred as measured at three months (graph #1). These levels invariably dropped in subsequent tests. This appears to indicate that there is a mobilization of stores of toxic metals in body tissues with this treatment protocol, which showed up as higher concentration in the hair with the three month analysis.
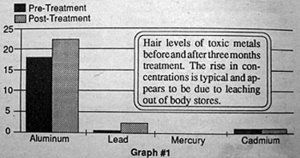
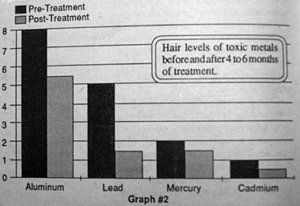
Repeat hair analysis at six to sixteen months has demonstrated reduced levels of toxic metal in the hair which appear to be time and dose related (graph #2 and 3).

Conclusions
A regimen of Formula ACX, taken three ounces per day is effective in reducing body loads of lead, mercury, cadmium, and aluminum, as evidenced by repeated hair analysis.
During the first three months of treatment, concentration of these metals in the hair will actually increase. During this period, treated individuals may actually feel a bit worse. It is interesting that this response may make the use of Formula ACX an effective screening method to determine latent body stores of toxic metals. Continued use would obviously complete the removal of such substances.